


Validation protocols are formal documents that define the plan, steps, and acceptance criteria for validating equipment, systems, or processes in regulated industries like pharmaceuticals, biotech, and manufacturing. Preparing these protocols and reports is crucial to ensure compliance and confirm that systems perform as intended.

In both the pharma and engineering sectors, efficient and reliable utility systems are essential for smooth operations, regulatory compliance, and safety. Our consultant excels in the design of utilities that are tailored to meet the specific needs of your projects, ensuring operational efficiency and adherence to industry standards.
Specializing in HVAC, compressed air, boilers, and pure steam systems for pharma and engineering sectors, ensuring efficient, compliant, and high-quality utility solutions
In pharma and engineering, recycling waste heat and water boosts efficiency, cuts costs, and supports sustainability. Our consultant designs systems that recover and reuse these resources, ensuring regulatory compliance and eco-friendly operations

In the pharma and engineering sectors, utilities like HVAC systems, compressed air systems, industrial boilers, and pure steam generators are vital components for maintaining controlled environments, ensuring product quality, and meeting regulatory requirements. Our consultant specializes in the design, installation, and optimization of these critical systems to ensure efficiency, safety, and compliance with industry standards.

In the pharma and engineering sectors, resource efficiency is key to optimizing operations, reducing costs, and minimizing environmental impact. One of the most effective strategies for achieving these goals is the recycling of wasted heat and water. Our consultant specializes in designing and implementing systems that capture and reuse waste heat and water, contributing to sustainable operations and compliance with environmental regulations.

In the pharma and engineering sectors, energy efficiency and power factor optimization are crucial for reducing operational costs, enhancing system reliability, and meeting regulatory standards. Our consultant specializes in energy saving strategies and power factor improvement solutions that not only help you lower energy consumption but also improve your bottom line and contribute to sustainability efforts.

In the pharma, medical devices and healthcare industries, ethylene oxide (EO) sterilization is a crucial process for ensuring the safety and sterility of products that cannot withstand traditional heat-based sterilization methods. Whether for pharmaceutical products, medical equipment, or packaging materials, our consultant specializes in designing, implementing, and optimizing Ethylene Oxide Sterilization Systems that adhere to strict regulatory standards and ensure high-quality sterilization outcomes.

In the pharma and engineering sectors, the reliability and efficiency of equipment are crucial to ensure continuous operations, product quality, and regulatory compliance. Our consultant specializes in creating Preventive Maintenance Checklists tailored to your specific systems and equipment, ensuring that every aspect of your operations is functioning at peak performance, minimizing downtime, and extending the lifespan of your critical assets.
In the pharma and engineering sectors, transformers are essential for ensuring the reliable and efficient distribution of electrical power to critical equipment and systems. However, inefficient transformer capacity can lead to energy wastage, increased operating costs, and potential overloading, which may impact operational continuity.

In the pharma and engineering sectors, high-voltage systems are critical for ensuring the reliable transmission and distribution of electrical power to large industrial machinery, production lines, and other critical infrastructure. Our consultant specializes in high-voltage system installations, providing safe, efficient, and compliant electrical solutions tailored to your specific needs. From designing the system to installation and testing, we ensure that your high-voltage systems operate optimally and safely.

In the pharma and engineering sectors, the installation of equipment and machinery is a critical aspect of ensuring smooth and efficient production processes. From high-tech pharmaceutical manufacturing lines to advanced engineering systems, proper installation ensures that your equipment operates at peak efficiency, meets regulatory standards, and integrates seamlessly into your existing operations. Our consultant specializes in equipment and machinery installations, offering expert support throughout the entire process, from design and planning to commissioning and ongoing support.

In both the pharma and engineering sectors, a Motor Control Centre (MCC) is a crucial component in controlling and managing electrical motors that drive critical machinery and equipment. Whether you're dealing with HVAC systems, production lines, compressors, or other motor-driven equipment, the MCC ensures that your motors operate efficiently, safely, and reliably. Our consultant specializes in the design, installation, and commissioning of MCC systems, providing tailored solutions that meet your specific operational needs and comply with industry standards.
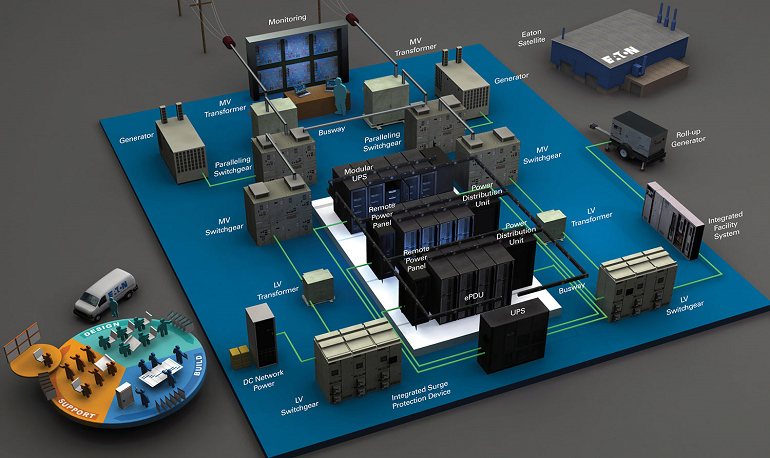
A power distribution system is the backbone of any industrial facility, ensuring that electricity is reliably and efficiently distributed to the critical equipment and machinery that drive operations. In the pharma and engineering sectors, a well-designed power distribution system is essential to ensure uninterrupted production, protect valuable assets, and comply with stringent safety and regulatory standards. Our consultant specializes in power distribution system design, offering tailored solutions that address the unique power needs of your facility while optimizing efficiency, safety, and reliability.

The design of electrical panels plays a crucial role in the efficiency, safety, and reliability of electrical systems in industries such as pharma and engineering. Electrical panels are responsible for distributing power to various equipment and protecting electrical circuits, ensuring that all electrical components function optimally and securely. Our consultant specializes in the design and engineering of electrical panels, providing tailored solutions to meet the specific requirements of your facility while ensuring compliance with industry standards and safety regulations.

Electrical safety is a critical aspect of any industrial or commercial facility, especially in high-risk environments such as the pharma and engineering sectors, where the safety of both personnel and equipment is paramount. A thorough Electrical Safety Audit is designed to identify potential hazards, ensure compliance with safety regulations, and improve the overall safety of electrical systems. Our consultant specializes in providing comprehensive Electrical Safety Audits, helping you minimize risks, enhance operational efficiency, and ensure the protection of both people and equipment.

In today's competitive business environment, optimizing energy usage is not only crucial for reducing costs but also for enhancing sustainability and complying with environmental regulations. Energy Saving Audits are comprehensive evaluations of a facility's energy consumption, helping businesses identify inefficiencies, reduce waste, and implement practical solutions for energy conservation. Our consultant specializes in Energy Saving Audits, providing actionable insights and solutions tailored to the unique needs of pharma and engineering sectors, where energy efficiency can significantly impact both operational costs and environmental footprint.

Maintaining high-quality standards and compliance is vital in the electrical industry, especially when dealing with safety, performance, and customer satisfaction. Our Internal & External Quality Systems / ISO Auditing Training prepares professionals to implement, evaluate, and audit quality management systems in alignment with ISO standards. Whether you're aiming to achieve certification or strengthen internal controls, this training helps ensure that your operations meet international benchmarks.

Environment, Health, and Safety (EHS) auditing is essential for ensuring a safe workplace, protecting the environment, and maintaining compliance with legal and industry standards. Our EHS Auditing Training is designed to equip safety officers, engineers, supervisors, and compliance professionals with the skills required to identify risks, evaluate existing controls, and implement continuous improvements in line with best practices and regulatory frameworks.

Process Safety Management (PSM) is a structured framework aimed at preventing the release of hazardous substances that could lead to fires, explosions, or toxic exposures in industrial settings. Our PSM Training is tailored for electrical, mechanical, and process professionals working in high-risk environments such as power plants, manufacturing units, and chemical facilities. This training provides the knowledge and tools to implement robust safety systems that protect people, assets, and the environment.